CLINICAL RESEARCH
The scientific literature supports key components of the programs, including the use of pre-packaged meals, low-GI foods, remote counseling, and self-monitoring of eating, activity, and progress. In addition to the broad scientific support for elements of the Nutrisystem programs, there is direct evidence in support of the Nutrisystem programs from clinical trials as well as analyses of our customers’ outcomes.
Recent Studies
- Hearty Inspirations Satiety Study – presented at the 2023 Food and Nutrition Conference & Expo, the annual conference of the Academy of Nutrition & Dietetics. Click here to read more: https://www.jandonline.org/article/S2212-2672(23)01340-0/fulltext
- ProSync shake Satiety Study – presented at Nutrition 2022, the annual conference of the American Society for Nutrition. Click here to read more: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9193336/
Clinical Trials
1. Foster GD et al. The effects of a commercially available weight loss program among obese patients with type 2 diabetes: a randomized study. Postgraduate medicine 2009; 121:113-18.
Click here to view abstract
2. Foster GD et al. A randomized comparison of a commercially available portion-controlled weight-loss intervention with a diabetes self-management education program. Nutrition and Diabetes 2013; 3:e63. : 10.1038/nutd.2013.3.
Click here to view abstract
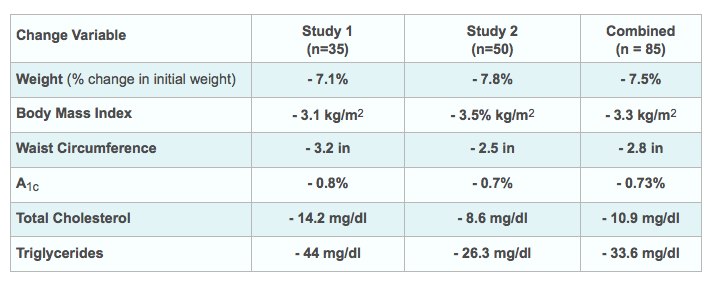
These two clinical trials examined the effects of D® on weight, hemoglobin A1C, and several additional metabolic outcomes among participants with type 2 diabetes. The 2009 study compared 3 months of Nutrisystem D, in conjunction with counseling sessions, to 3 months of information and support sessions about diabetes management. In months 4-6, all participants received Nutrisystem D. Patients on insulin were excluded from this study. The 2013 study, which included participants on insulin, compared Nutrisystem D, in conjunction with counseling sessions, to structured diabetes self-management education. Both interventions were delivered in 9 sessions over 6 months.
The table below summarizes the mean 6-month changes from baseline among all participants randomized to receive the Nutrisystem D intervention in both trials, separately and combined. These data are from an unpublished secondary analysis of within-group changes observed in the intent-to-treat samples of the two trials. All changes from baseline reported here are statistically significant (p < 0.05).
As shown in the figure below, more than half of Nutrisystem D participants in the two studies combined achieved clinically meaningful changes in weight and A1C. Among persons who entered the study with A1C > 7.0%, more than half achieved A1C < 7.0% at 6 months.
Percentage of Participants
These findings suggest both statistically and clinically meaningful reductions in body weight and A1C among participants who received the Nutrisystem D intervention, along with counseling. As detailed below, the weight losses observed in the clinical trials are corroborated by real-world customer results.
3. Fabricatore AN et al. Reduction in glycemic variability and hyperglycemia with a low-glycemic index portion-controlled diet in persons with type 2 diabetes. Presented at the 72nd Annual Scientific Sessions of the American Diabetes Association. Philadelphia, PA June 10, 2012.
This randomized cross-over trial employed continuous glucose monitoring (CGM) to assess multiple indicators of glycemic variability and stability in obese adults with type 2 diabetes. Subjects served as their own controls, consuming their usual diet and Nutrisystem D, in random order, for two weeks each, separated by a one-week washout period. During consumption of Nutrisystem D, participants had significantly lower average blood glucose (137.2 vs. 148.9 mg/dl, p < 0.01) and significantly less glycemic variability, as measured by standard deviation (31.8 vs. 36.1 mg/dl, p < 0.04) and interquartile range (38.9 vs. 45.0 mg/dl, p < 0.05) of blood glucose values. Additionally, participants recorded significantly fewer values in the hyperglycemic range (i.e., > 180 mg/dl; 13.4% vs. 21.0% of values, p < 0.03) without a significant increase in hypoglycemic values (i.e., < 70 mg/dl; 3.3% vs. 1.3%, p = 0.19). The figure below shows subjects’ average blood glucose tracings during consumption of the portion-controlled diet (i.e., Nutrisystem D) versus their usual diet.
4. Clinical study conducted by Biofortis Clinical Research, sponsored by Nutrisystem. Comparison of Commercially-Available Portion Controlled Weight Loss Programs with a Self-Directed Diet: A Randomized Trial
Previous research has shown that provision of meals and use of meal-replacement products promotes greater weight loss compared to standard reduced calorie diets.
Objective: The aim of this randomized, parallel group study was to examine the magnitude of changes in body weight, body circumference, and related outcomes achievable when generally healthy overweight and obese adults followed different formats of a commercially-available structured weight loss program providing portion-controlled foods, compared to a self-directed diet.
Methods: 161 overweight/obese adults (n = 123 women, n = 38 men) with a mean (± standard error of the mean) age of 50.2 ± 0.9 y and body mass index (BMI) of 33.5 ± 0.3 kg/m2 at screening were randomized (stratified by BMI and age categories) to a self-directed diet or one of three Nutrisystem® programs: 1) My Way; 2) Turbo 10; or 3) a program modeled after the Dietary Approaches to Stopping Hypertension (DASH) diet. Daily energy intake targets for each intervention were 1500 kcal/day (men) or 1200 kcal/day (women), except for the first week of the Turbo 10 intervention, which included a more aggressive 1000 kcal/day diet to start. Changes in body weight, total body circumference (chest + waist + hip + dominant arm + dominant thigh), and body fat mass (measured via dual energy x-ray absorptiometry) from baseline to week 4 were outcomes of primary interest. Results presented were derived from a modified intent-to-treat sample with last-observation carried forward in a repeated measures analysis of covariance model.
Results: As shown in the table below, subjects in each intervention experienced a statistically significant average change in body weight (p ≤ 0.0007 for all), total body circumference (p ≤ 0.0005 for all), and body fat mass (p ≤ 0.0165 for all) at 4 weeks. These changes were also statistically significantly different for each Nutrisystem intervention compared to the self-directed diet (p ≤ 0.0054 for all). Additionally, 39.5% and 26.8% of the subjects in the Turbo 10 and DASH interventions, respectively, lost ≥5 % of initial body weight at 4 weeks compared to 7.9% of subjects assigned to the self-directed diet (p ≤ 0.0326 for each comparison).
Conclusion: Each Nutrisystem intervention resulted in meaningful changes in body weight, total body circumference, and body fat mass over a 4-week period, compared to a self-directed diet
Customer Studies
1. Daggy BP et al. Trial participants and paying customers achieved similar weight losses with a commercial weight loss program for type 2 diabetes. Diabetes 2011; 60 (Suppl. 1); A702.
Nutrisystem researchers attempted to determine whether the results of the Foster et al. (2009) clinical trial were representative of the outcomes achieved by actual Nutrisystem D customers who were not participating in a structured trial. Nutrisystem D customers who used Nutrisystem’s online weight-tracking tool to record their weight at baseline (i.e., time of initial purchase) and three months later (i.e., to match the length of the intervention in the clinical trial) were included in the analysis. The sample of 5,588 customers (64% women, mean age = 52.5 y, mean weight = 107.5 kg, mean BMI = 37.6 kg/m2) achieved an average 3-month weight loss of 8.2 kg, which represented a 7.6% reduction in initial body weight. By comparison, participants in the Foster et al. trial also lost 8.2 kg, representing a 7.1% weight loss. Approximately two-thirds of trial participants (67.7%) lost at least 5% of their initial weight, compared with over three-quarters (78.4%) of customers who reached that target. Thus, it appears that actual Nutrisystem D customers achieved similar weight losses to those achieved in a clinical trial.
2. Fabricatore AN et al. Results not typical? Subjective and objective success in a commercial weight loss program.Presented at the Annual Scientific Meeting ofThe Obesity Society. Orlando, FL. October 2, 2011.
Advertisements and testimonials for commercial weight loss programs (including Nutrisystem) often depict dramatic results that must be disclaimed as “not typical.” This study attempted to define the typical results achieved by Nutrisystem customers. The only criterion for inclusion in the analysis was that the customer recorded their weight at baseline and at 3 months, which yielded a sample of 103,693 customers (70% women, mean age 46.9 y, mean weight = 216.4 lb, mean BMI = 34.3 kg/m2). The average weight loss at 3 months was 18.2 lb, representing an 8.3% reduction in initial weight. Although few customers (4.5%) reached their self-selected weight loss goal (mean goal = 54 lb loss) at 3 months, nearly four-fifths (79.4%) achieved at least a 5% weight loss in 3 months, including 33% of customers who lost 10% or more of their starting weight. This analysis suggested that the typical result for Nutrisystem customers who maintained some level of engagement for 3 months was a clinically meaningful weight loss.
3. Daggy BP et al. Holiday weight change in a commercial weight loss program. Presented at Advances and Controversies in Clinical Nutrition (a meeting of the American Society for Nutrition). San Francisco, CA. February 26, 2011.
The period spanning Thanksgiving to New Year’s Day is a particularly difficult time for weight control. Nutrisystem researchers sought to examine how program customers fared during that time. Customers who recorded their weight in the week before Thanksgiving and again in the week following New Year’s Day were included in the analysis. The average weight loss achieved during that 6-week period was 5.5 lb. For comparison purposes, weight changes during similar 6-week periods (beginning 3, 6, and 9 months prior to Thanksgiving) were examined and found to range from 8.4 to 9.5 lb. Thus, although customers lost less weight during the holiday season than during other times of the year, the average result was a loss of nearly 1 lb. per week during that traditionally difficult period for weight control.
4. Figueroa et al. Effects of hypocaloric diet, low-intensity resistance exercise with slow movement, or both on aortic hemodynamics and muscle mass in obese postmenopausal women. Menopause. 2013; 20 (9): 000-000. Click here to view abstract.
This randomized controlled clinical trial examined the independent and combined effects of a low-calorie Nutrisystem diet and low-intensity resistance exercise training (LIRET) on aortic hemodynamics and appendicular skeletal muscle mass (ASM). Forty-one sedentary, postmenopausal women with obesity were randomly assigned to one of three interventions — (1) Nutrisystem diet only, (2) LIRET only, or (3) Nutrisystem diet combined with LIRET. After 12 weeks, significant decreases in body weight (-5.7 [1.0] and -4.9 [1.0] kg), BMI (-2.3 [0.4] and -1.9 [0.4] kg/m2), and waist circumference (-7 [2] and -5 [1] cm) were seen in the diet and diet + LIRET groups (respectively). Body weight reductions in both diet groups, as well as BMI and waist circumference reductions in the diet group, were significant compared with the LIRET group. ASM index was significantly increased only after diet + LIRET (0.9% [0.3%]), but there was no significant group-by-time interaction. In the LIRET, diet, and diet + LIRET groups (respectively), clinical significant decreases were observed for brachial and aortic SBP (-7 [3], -7 [2], and -9 [2] mm Hg), aortic P1 (-6 [2], -7 [2], and -7 [3] mm Hg), and aortic P2 (-7 [3], -7 [2], and -9 [2]mm Hg). Brachial and aortic DBP significantly decreased (-6[2] mm Hg) only in the diet + LIRET group. This study shows that 12 weeks of Nutrisystem hypocaloric diet with and without LIRET improve aortic BP in obese postmenopausal women. Although LIRET does not confer additional benefit on aortic hemodynamics, LIRET prevents loss of ASM during caloric restriction in obese postmenopausal women.
Nutrisystem Supports Clinical Research
Nutrisystem is proud to partner with a number of academic institutions to support clinical investigations that include products and programs. Support mechanisms include investigator-initiated grants, in-kind product donations, and access to de-identified data.